Infliximab Blood Monitoring
blood infliximab monitoring wallpaperInfliximab Remicade Renflexis Inflectra is a chimeric immunoglobulin IgG1 kappa targeting tumor necrosis factor-alpha TNF-a and it is currently FDA-approved for the treatment of multiple inflammatory conditions. All patients should be observed carefully for 12 hours after infusion and resuscitation equipment should be available for immediate use risk of hypersensitivity reactions.
 Procisedx Achieves Ce Mark For Infliximab And Adalimumab Point Of Care Testing
Procisedx Achieves Ce Mark For Infliximab And Adalimumab Point Of Care Testing
Suggested monitoring guidelines The clinician must weigh the potential clinical benefits of TNF inhibition against potential adverse effects.

Infliximab blood monitoring. In this setting TDM helps identify patients likely to benefit from dose intensification. Monitor for infection before during and for 6 months after treatment. However different hospitals may do this differently and some hospitals allow patients to go home as soon as the infusion has finished.
The clinical utility of pre-emptive testing to maintain drug levels within a therapeutic range is currently uncertain although its use in this setting is increasing in clinical practice. If blood cell counts get very low this can lead to bleeding problems infections or anemia. Infliximab monitoring assays from Quest Diagnostics were originally designed to detect the reference drug infliximab and its anti-drug antibody Inflectra infliximab-dyyb an FDA-approved biosimilar of infliximab exact same amino acid sequence has become commercially available.
Low blood cell counts have happened with infliximab injection. Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with infliximab including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy. The most promising indication for TDM is the assessment and management of treatment failure reactive testing.
It is important to warn patients of all serious adverse effects and to check their complete blood count as required eg in the event of fever anemic symptoms bruising and easy bleeding. It is recommended that patients on biologic medications have routine blood tests every 6 months or so including full blood count and liver function tests. Written evidence of Infliximab administration is recorded in the patients medical notes MDT notes.
An active infliximab trough monitoring strategy should be implemented with automatic measurement of drug antibodies in low trough levels. Adalimumab Humira is administered by subcutaneous injection by a nurse or by the patients themselves. Sometimes these have been deadly.
Acute infusion reactions including anaphylactic reactions may develop within seconds or within a few hours following infusion. Out patients Follow- Up All patients receiving Infliximab will be reviewed in out patients clinic after every 3. Inflectra and Remsima is administered by means of IV infusion in a hospital setting.
Adequate infliximab IFX levels are associated with favorable outcomes in inflammatory bowel disease. Low-serum IFX levels are associated with the development of antibodies to IFX ATI which subsequently associated with clinical relapse and increased morbidity. The recommended dose is 5mg of infliximab for every kilogram you weigh.
A l - though specific laboratory monitoring is not currently mandated the frequen - cy and chronology of cytopenias and hepatotoxicity warrants that a complete blood count and liver function tests be. The presence of infliximab drug even at concentrations well above target treatment levels 50 ugmL does not interfere with the anti-infliximab antibody detection. Ongoing monitoring The above nursing assessment and monitoring is repeated for every admission for Infliximab by the nursing staff.
Monitoring while on infliximab Regular follow-up visits to monitor the safety and efficacy of treatment are necessary. It is unknown whether intermittent monitoring of complete blood counts when the patient is taking infliximab can prevent serious outcomes. Monitor for symptoms of delayed hypersensitivity if re-administered after a prolonged period.
TB tests should be repeated from time to time. Infliximab Remicade and its biosimilars. The hospital will work out how much you need and make up the infusion for you so.
WHAT IS THE NORMAL DOSAGE. Therapeutic drug monitoring TDM of anti-TNF therapies Infliximab and Adalimumab. With such practice patients with incomplete therapeutic response will have timely escalation of the therapy and possibly less drug antibody formation.
Infliximab IFX is an effective treatment for the management of moderate to severe inflammatory bowel disease IBD. Infliximab binds to soluble TNF-a and transmembrane homotrimers which are found on the surface of macrophages and T-cells with similar affinity. This test 1 measures infliximab and infliximab-dyyb inflectra levels to help differentiate pharmacodynamic or pharmacokinetic conditions to guide therapy and 2 tests for anti-drug antibodies to help determine if changing the treatment plan is appropriate.
Using therapeutic drug monitoring TDM to guide dosing is cost effective and associated with clinical improvement but effect on endoscopic outcomes remains unclear. In the presence of anti-infliximab antibodies the infliximab drug level typically reflects the antibody-unbound fraction of infliximab concentration in serum. The American Gastroenterological Association AGA recommends optimal infliximab trough.
Primary responders to IFX who underwent dose escalation 2008-2014 were reviewed. Monitoring of Infusion Infliximab has been associated with acute infusion-related reaction including anaphylactic shock and delayed hypersensitivity reactions. If you have questions talk with the doctor.
The blood sample should be collected before a new injection. The primary purpose of this study is to examine the relation between dose and interval to IFX level.
 Therapeutic Drug Monitoring Of Infliximab In Spondyloarthritis A Review Of The Literature Fobelo Lozano 2019 British Journal Of Clinical Pharmacology Wiley Online Library
Therapeutic Drug Monitoring Of Infliximab In Spondyloarthritis A Review Of The Literature Fobelo Lozano 2019 British Journal Of Clinical Pharmacology Wiley Online Library
 Drug Monitoring Of Infliximab Biosimilars En Clinical Diagnostics
Drug Monitoring Of Infliximab Biosimilars En Clinical Diagnostics
 Ridascreen Ifx Monitoring En Clinical Diagnostics
Ridascreen Ifx Monitoring En Clinical Diagnostics
 Available Now The Complete Package For Therapeutic Drug Monitoring
Available Now The Complete Package For Therapeutic Drug Monitoring
 Pdf Infliximab Concentration Monitoring Improves The Control Of Disease Activity In Rheumatoid Arthritis
Pdf Infliximab Concentration Monitoring Improves The Control Of Disease Activity In Rheumatoid Arthritis
Https Academic Oup Com Ecco Jcc Article Pdf 11 Suppl 1 S378 10450886 Jjx002 709 Pdf
 Clinical Efficacy Of Infliximab Level And Anti Infliximab Antibody Measurement In Patients With Inflammatory Bowel Disease An Audit Springerlink
Clinical Efficacy Of Infliximab Level And Anti Infliximab Antibody Measurement In Patients With Inflammatory Bowel Disease An Audit Springerlink
 Algorithm For Tdm Guided Decision Making For Early Optimization Of Download Scientific Diagram
Algorithm For Tdm Guided Decision Making For Early Optimization Of Download Scientific Diagram
 An Automated Mass Spectrometric Blood Test For Therapeutic Drug Monitoring Of Infliximab Sciencedirect
An Automated Mass Spectrometric Blood Test For Therapeutic Drug Monitoring Of Infliximab Sciencedirect
Https Www Nice Org Uk Advice Mib109 Resources Ridascreen Tests For Monitoring Infliximab In Inflammatory Bowel Disease Pdf 2285963222697925
 Pdf Annals Express Therapeutic Drug Monitoring Of Tnf Inhibitors In The Management Of Chronic Inflammatory Diseases
Pdf Annals Express Therapeutic Drug Monitoring Of Tnf Inhibitors In The Management Of Chronic Inflammatory Diseases
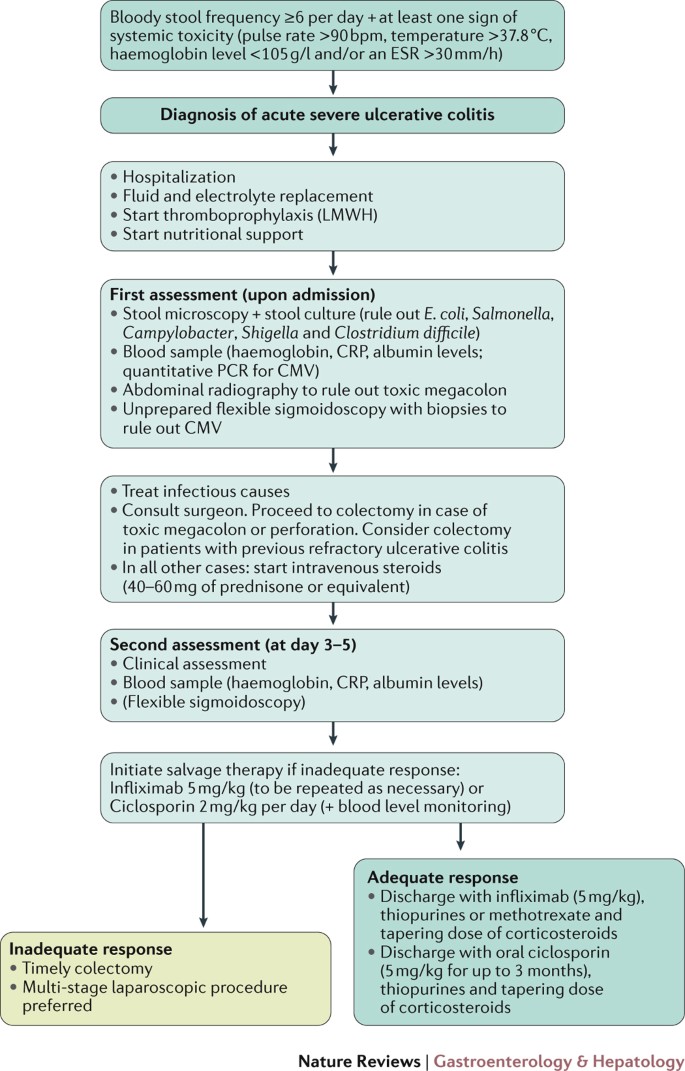 Acute Severe Ulcerative Colitis From Pathophysiology To Clinical Management Nature Reviews Gastroenterology Hepatology
Acute Severe Ulcerative Colitis From Pathophysiology To Clinical Management Nature Reviews Gastroenterology Hepatology
Https Www Svhm Org Au Articledocuments 2305 Infliximab Pdf Aspx Embed Y
 Pdf Infliximab Pharmacokinetics In Inflammatory Bowel Disease Patients
Pdf Infliximab Pharmacokinetics In Inflammatory Bowel Disease Patients
Https Surreyandsussex Nhs Uk Wp Content Uploads 2013 04 Ibd Infliximab Protocol Pdf
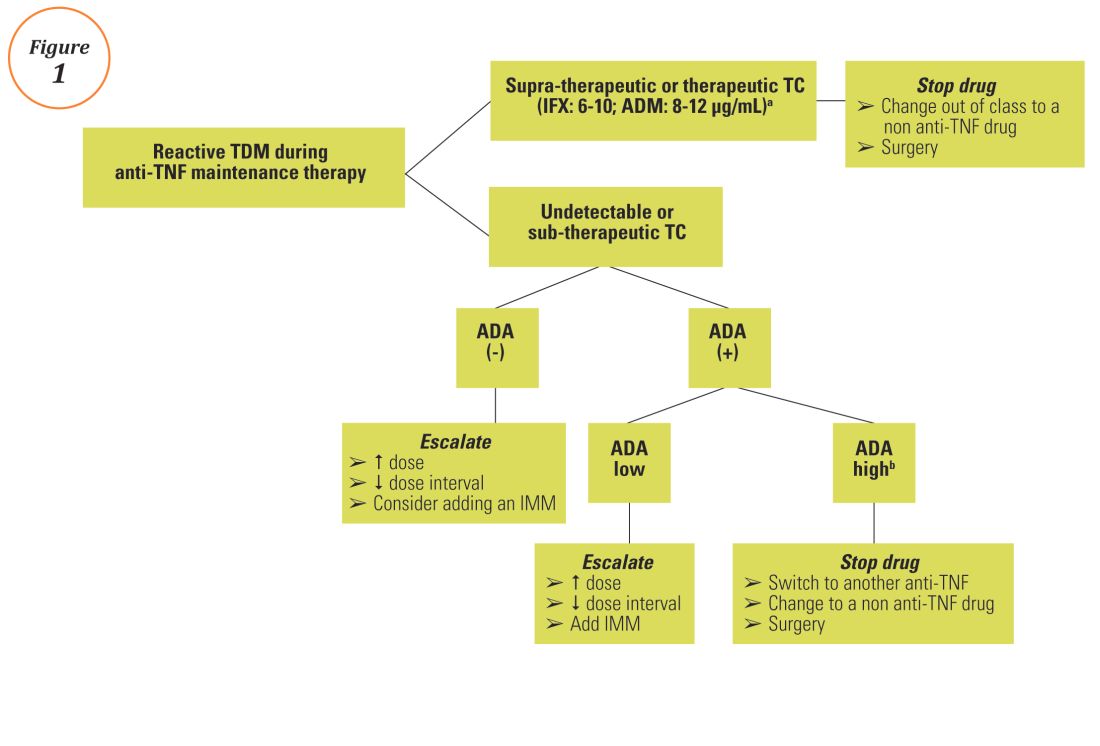 The Nuts And Bolts Of Drug Concentration Monitoring In Ibd Gi And Hepatology News
The Nuts And Bolts Of Drug Concentration Monitoring In Ibd Gi And Hepatology News
 Proteins And Antibodies Fox Biosystems
Proteins And Antibodies Fox Biosystems
 The Incidence And Management Of Infusion Reactions To Infliximab A Large Center Experience Sciencedirect
The Incidence And Management Of Infusion Reactions To Infliximab A Large Center Experience Sciencedirect